electron configuration chart|electron configuration chart periodic table : Pilipinas Hun 14, 2015 Jesus Slots is on Facebook. Join Facebook to connect with Jesus Slots and others you may know. Facebook gives people the power to share and makes the.
electron configuration chart,Mar 23, 2023 Click on above elements (in Periodic table) to see their information or Visit .
Electron Configurations of Atoms of Elements. The electron configuration of an atom of any element is the of electrons .
Learn how to write electron configurations using standard and abbreviated notations, and how to use orbital diagrams to visualize them. Find examples of e. Hun 14, 2015
electron configuration chart electron configuration chart periodic tableUse the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Given: series of elements. Asked for: valence .A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical .Electron configurations of the elements (data page) This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the .The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .The easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is .
Periodic table (electron configurations) Configurations of elements 109 and above are not available. Predictions from reliable sources have been used for these elements. Grayed .
The electron configuration of argon is 1s 2 2s 2 2p 6 3s 2 3p 6, and thus the abbreviated notation of potassium is written as [Ar] 4s 1. Similarly, the electronic configuration of argon is written using its .
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic .So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. . The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to find the highest electron orbital. Alternatively, remember group 1 (alkali metals) and group 2 (alkaline earth metals) are s-block, groups .The easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. It looks something like this.
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .Such overlaps continue to occur frequently as we move up the chart. Figure 6.24 Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). . When their electron configurations are added to the table (Figure 6.29), we also see a periodic recurrence of similar electron configurations in the outer .Periodic table (electron configurations) Configurations of elements 109 and above are not available. Predictions from reliable sources have been used for these elements. Grayed out electron numbers indicate subshells filled to their maximum. Bracketed noble gas symbols on the left represent inner configurations that are the same in each period.
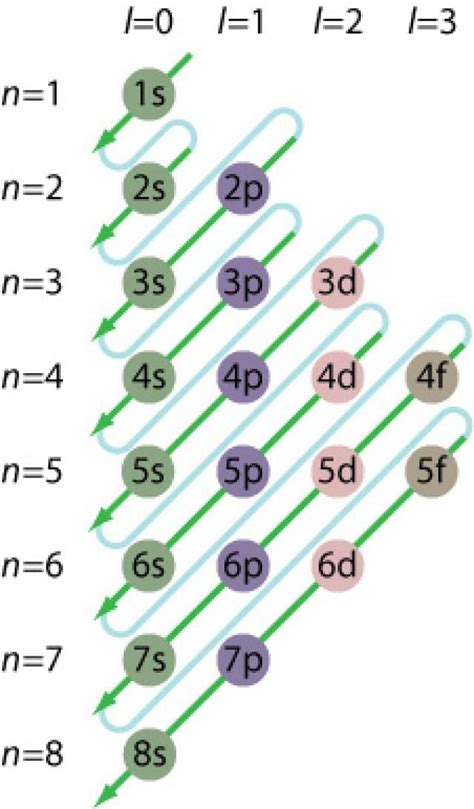
An electron configuration chart of the elements shows the periodicity of the electron structure across the elements. Electron Configuration Explained. In this chart, the numbers (1, 2, 3,.) are referred to as the principal quantum number, referred to as n, which corresponds to an electron shell. So, 1 refers to the first shell, 2 the second . Introduction to electron configurations. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of .Two of the lithium electrons can fit into the 1 s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2 s orbital. Therefore, we write the electron configuration of a lithium atom as 1s22s1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure 2.7.1 2.7. 1 ).The electron configurations of the elements are in Figure 6.9.2. Because each orbital can have a maximum of 2 electrons, there are 2 columns in the s block, 6 columns in the p block, 10 columns in the d block, and 14 columns in the f block. Hydrogen and helium are placed somewhat arbitrarily. Although hydrogen is not an alkali metal, its 1 s1 .
electron configuration chartThe electron configurations of a few elements are provided with illustrations in this subsection. Electron Configuration of Hydrogen. The atomic number of hydrogen is 1. Therefore, a hydrogen atom contains . The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .
electron configuration chart periodic tableIts electronic configuration is 1s2, 2s2, 2p6, 3s1 The largest value of the Principle Quantum Number (n) is 3, so that is the outermost orbital. Counting the number of electrons, we find that only the s orbital is present and it has only one electron. So Na has one electron in its outermost orbital.

The electron configuration of boron is: B: 1s 2 2s 2 2p 1. Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. The electron configurations of elements with higher atomic number can be written by following the orbital-filling chart in Figure 5.9.
Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from .
In several cases, the ground state electron configurations are different from those predicted by Figure 6.8.1 6.8. 1. Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s1 3 d5 rather than the predicted [Ar]4 s2 3 d4.
electron configuration chart|electron configuration chart periodic table
PH0 · which element has this electron configuration
PH1 · printable electron configuration chart
PH2 · periodic table of elements with electron configuration
PH3 · electron configuration to element calculator
PH4 · electron configuration for every element
PH5 · electron configuration chart periodic table
PH6 · electron configuration chart pdf
PH7 · electron configuration chart calculator
PH8 · Iba pa